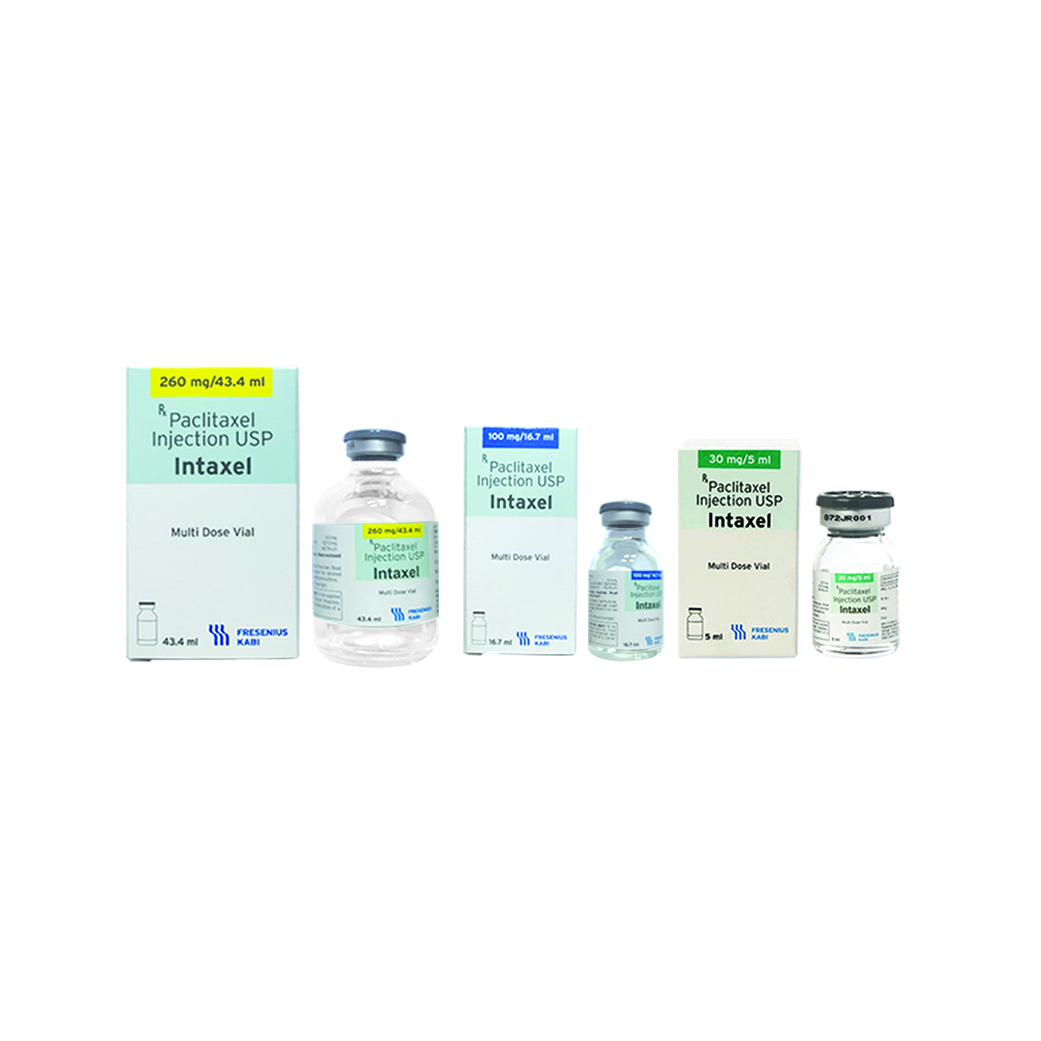
- Intaxel - Paclitaxel 30 mg/5 ml
- Intaxel - Paclitaxel 100 mg/16.7 ml
- Intaxel - Paclitaxel 260mg/43.4 ml
All patients should be premedicated prior to INTAXEL administration in order to prevent severe hypersensitivity reactions. (See ‘Premedication Regimens’ below).
Ovarian Cancer:
For previously untreated patients with carcinoma of the ovary, one of the following recommended regimens may be given every 3 weeks.
Paclitaxel administered intravenously over 3 hours at a dose of 175 mg/m2 followed by cisplatin at a dose of 75 mg/m2;
Paclitaxel administered intravenously over 24 hours at a dose of 135 mg/m2 followed by cisplatin at a dose of 75 mg/m2
In patients previously treated with chemotherapy for carcinoma of the ovary, paclitaxel Injection has been used at several doses and schedules; however, the optimal regimen is not yet clear. The recommended regimen is paclitaxel 135 mg/m2 or 175 mg/m2 administered intravenously over 3 hours every 3 weeks.
Breast Cancer:
For the adjuvant treatment of node-positive breast cancer, the recommended regimen is paclitaxel, at a dose of 175 mg/m2 intravenously over 3 hours every 3 weeks for four courses administered sequentially to doxorubicin-containing combination chemotherapy. The clinical trial used four courses of doxorubicin and cyclophosphamide.
After failure of initial chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy, paclitaxel at a dose of 175 mg/m2 administered intravenously over 3 hours every 3 weeks has been shown to be effective.
Non-Small Cell Lung Cancer:
For patients with non-small cell lung carcinoma, the recommended regimen, given every 3 weeks, is paclitaxel administered intravenously over 24 hours at a dose of 135 mg/m2 followed by cisplatin, 75 mg/m2.
AIDS-related Kaposi’s Sarcoma:
For patients with AIDS-related Kaposi’s sarcoma, paclitaxel administered at a dose of 135 mg/m2 given intravenously over 3 hours every 3 weeks or at a dose of 100 mg/m2 given intravenously over 3 hours every 2 weeks is recommended (dose intensity 45-50 mg/m2/week